DetailedConceptExplanation
Freezingpointandmeltingpointaresynonymous,referringtothetemperatureatwhichasubstancecoexistsinaliquidandasolidstate,orasubstanceinaliquidstateandasolidstateThetemperaturewhenswitchingbetween.Onlythefreezingpointormeltingpointofwateriscalledfreezingpoint,andthefreezingpointormeltingpointofothersubstancescannotbecalledfreezingpoint.
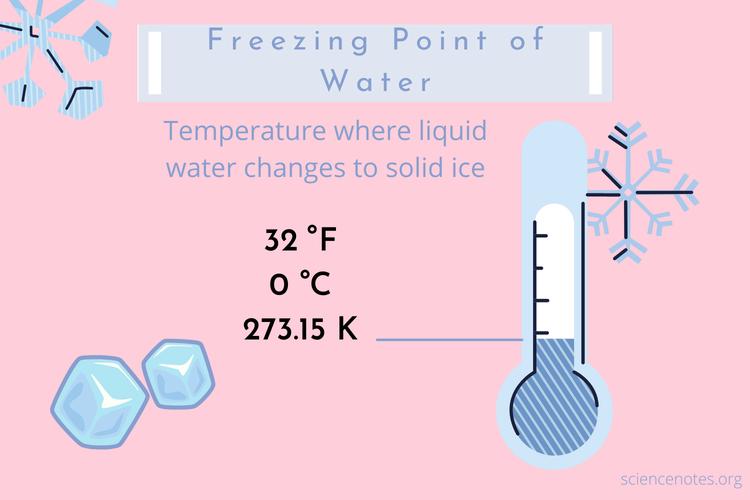
Usually,freezingpointreferstothefreezingpointofwateratastandardatmosphericpressure(1.013×10^5Pa),whichis0℃(273K).Underdifferentatmosphericpressures,thefreezingpointisdifferent.Ifyouwanttoestimatethefreezingpointatacertainpressure,youcanfindtheanswerfromthephasediagramofwater.Itisworthnotingthatthefreezingpointofwateratastandardatmosphericpressureisnotequaltothetriplepointofwater-thedifferencebetweenthetwois0.01K.
Thefreezingpointisalsorelatedtothepurityofthewater.Thefreezingpointofpurewateratstandardatmosphericpressureis0℃,butwhenthewatercontainsimpurities,thefreezingpointwilldecrease.Forexample,thefreezingpointofseawaterislowerthanthatoffreshwater.Thefreezingpointofseawateriscloselyrelatedtothesalinityofseawater.Whenthesalinityreaches24.695,thefreezingpointofseawaterisonly-1.332℃.
Application
CalibratingThermometer
Thefreezingpointcanbeusedtocalibratethereadingofthethermometer.Becausethefreezingpointisconstantatagivenairpressure,thetemperatureoftheice-watermixtureundertheairpressurecanbeusedtocalibratethereadingoftheprecisionthermometer.Manyinstrumentcompanieshavedevelopedinstrumentsforcalibratingthermometers-freezingpointreferencechambersbasedonthisprinciple.Thefreezingpointreferencechamberusuallyusesthefreezingpointunderastandardatmosphericpressure,whichis0℃,tocalibratethereadingofaprecisionthermometer.
Freezingpointreductiondatamethod
Wecanknowtheconcentrationofdissolvedmatterintheaqueoussolutionthroughthefreezingpointoftheaqueoussolution.Whenthewatercontainsdissolvedsubstances,thefreezingpointwilldecrease.Thehighertheconcentrationofdissolvedmatter,thelowerthefreezingpoint.Thefreezingpointordissolvedconcentrationoftheaqueoussolutioncanbecalculatedfromtheformula:Intheformulaisthedifferencebetweenthefreezingpointofpurewater(0℃)andthefreezingpointofthesolutionatstandardatmosphericpressure,andKiswaterMolarfreezingpointdepressionconstant(itsvalueis1.86K·Kg/mol),bisthemassmolarconcentrationofthedissolvedmatter.
Intheuseofdruginjections,theinjectionmustbeclosetotheosmoticpressurevalueoftheserum(usuallyabout285mosm/kg)inordertoreducethediscomfortduringinjection.Thefreezingpointreductiondatamethodisacommonlyusedmethodofosmoticpressureadjustment.Undernormalcircumstances,thefreezingpointofplasmais-0.52℃.Whenthefreezingpointoftheaqueoussolutiondropsto-0.52℃,itisisotonicwithplasma.ThedosageofisotonicregulatorcanbedirectlycalculatedbytheformulaW=(0.52-a)/b(thisformulaisderivedfromtheaboveformula).Amongthem,Wisthepercentageofisotonicregulatortobeaddedtopreparetheisotonicsolution,aisthedegreeoffreezingpointdepressionofthedrugsolution,andbisthedegreeoffreezingpointdepressionofthe1%solutionofisotonicagentusedtoadjust.Calculatethedosageofisotonicregulator,andthenformulateaninjectionthatisisotonicwithplasma.Thisisthefreezingpointreductiondatamethod.
